“Almost Perfect” Equations of State and Adiabatic Exponents#
Friday, Jan. 31, 2025
astrophysics of stars and planets - spring 2025 - university of arizona, steward observatory
Today’s Agenda#
Announcements - Grades,Volunteers for Solutions, Uploading HW2 (2m)
Reading Overview/Key Points (10m)
In-Class HW1 Review (Groups of ~4) (20m)
In-Class HW1 Presentations (Groups of ~4) (15m)
Debrief/Reminders (2m)
“Almost Perfect” Equations of State#
In real gases, interactions have to be taken into account that modify the “perfect” results given above. In addition, a stellar equation of state might consist of many components with radiation, Maxwell–Boltzmann, and degenerate gases competing in importance.
A measure of the interaction energy between two ions is the Coulomb potential
Definition 29
Coulomb potential between two ions
where \(a\) is the typical seperation between the two ions. Similar to past estiamtes, we suggest that these effects become important when they are comparable to the thermal energy \(kT\). This allows us to define the ratio,
Definition 30
Plotting the different regimes we find:
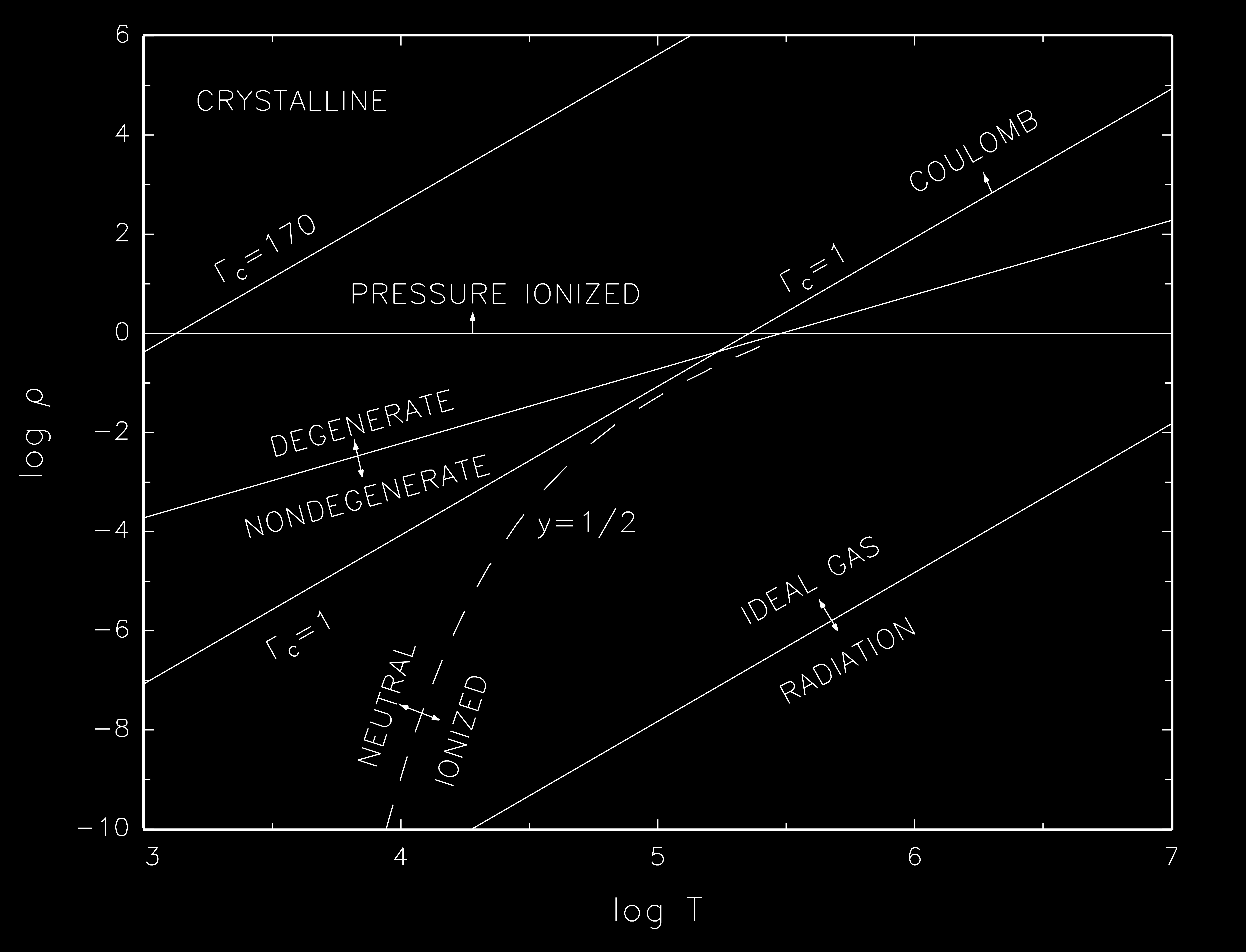
A composite showing how the ρ–T plane is broken up into regions dom- inated by pressure ionization, degeneracy, radiation, ideal gas, crystallization, and ionization-recombination. The gas is assumed to be pure hydrogen. From HKT 3.9.
Observation 4
The axis are flipped, \(\rm{log}T\) on the x-axis in this example!
Roughly, for \(\Gamma_{v}\gt1\) Coulumb effects may be important. For values of \(\Gamma_{v}\gt170\) and appropriate densities such as in white dwarfs, Coulomb effects overwhelm those of thermal agitation and the gas settles down into a crystal. Also noted is the boundary at which the Saha equation assumptions terminate - pressure ionization.
Adiabatic Exponents and Other Derivatives#
3.7.1 Keeping the Composition Fixed#
Specific Heats#
Definition 31
general form of specific heat
where \(\alpha\) is kept fixed as \(T\) changes.
Units
\(Q\) - (\(\rm{erg \ g}^{-1}\))
\(c_{\alpha}\) - (\(\rm{erg \ g^{-1} K^{-1}}\))
From HKT Eq. 1. we have:
Definition 32
where \(V_{\rho} = 1/\rho\) is the specific volume.
This leads us to
Definition 33
the specific heat at constant-volume
for an ideal monotomic gas we have \(E=3N_{\rm{A}}kT/2\mu\) such that \(c_{V_{\rho}}=3N_{\rm{A}}k/2\mu\) or \(E=c_{V_{\rho}} T\).
To find the specific heat at constant pressure we start with the relation:
Definition 34
Relation between specific heats
using the power-law expression for pressure from HKT 1.67, \(P = P_{0}\rho^{\chi_{\rho}}T^{\chi_{T}}\).
This gives the following definitions for the constants \(\chi_{T}\) and \(\chi_{\rho}\):
Definition 35
and
Definition 36
Finally, combining this all together we find a reduced expression:
Definition 37
Simplified relation between specific heats
For an Ideal Gas, \(\chi_{T}=\chi_{\rho}=1\) giving
and finally \(c_{P}=5N_{\rm{A}}k/2\mu\).
We also define a new variable
Definition 38
Ratio of specific heats
Adiabatic Exponents#
The dimensionless adiabatic exponents, the \(\Gamma\)’s measure the thermodynamic response of the system to adiabatic changes and will be used extensively. As in Chapter 1, the subscript “ad” means that the indicated partials are to be evaluated at constant entropy.
Definition 39
Definition 40
Definition 41
Finally, we have
Now, we can write some more useful versions of these exponents and relating to the specific heats:
Definition 42
Definition 43
Definition 44
and lastly, \(\gamma\)
Definition 45
Mixtures of Ideal Gases and Radiation#
For stellar models, an equation of state that is a mixture of ideal gas and radiation can suffice, allowing us to write the pressure as:
Definition 46
and the energy in a similar fashion
Definition 47
Observation 5
Be sure to keep track of \(V\) and \(V_{\rho}\) when using any of the above equations!
In-Class Activity#
Form groups of 3-4
Choose a problem from the HW1 (Exercise 1 or 2).
Compare solutions, nominate a scribe that will write up the solutions and that you all agree on the answer!
This person will have 5 minutes at the end class to share a condensed version of the solution for the class.
Learning Objective#
discuss exercises with others to see if you agree with the logic / steps OR identify where your steps diverge


